Back to the Beginning
Really understanding the difference between backdrafts and smoke explosions requires an understanding of several basic concepts of fire dynamics. Much of this should be a review, but there are several important connections to the phenomena of backdraft and smoke explosion.

Combustion
Combustion is a chemical process where a fuel reacts with an oxidizer, usually atmospheric oxygen, releasing the fuel’s chemical potential energy as thermal energy. In most cases fuel needs to be heated to its ignition temperature for combustion to begin.
Flaming combustion and surface combustion are both oxidation reactions releasing thermal energy but are distinguished by the location of the chemical reaction: flaming combustion occurs in the gas phase above the fuel, while surface (heterogeneous) combustion happens on the fuel’s surface.
To have flaming combustion, the fuel needs to be in the gas phase. For flammable gases it is simple, they are already there. Liquid fuels must be vaporized, and solid fuels must be pyrolyzed and/or melted and vaporized (in the case of thermoplastics). In examining the phenomena of backdraft and smoke explosion, we are most interested in typical building contents and combustible structural materials; hydrocarbon-based synthetic fuels (e.g., plastics) and cellulose-based fuels (e.g., wood).
Pyrolysis is a thermochemical decomposition process where organic materials break down into simpler, often volatile compounds when heated. It is important to understand that pyrolysis can occur in the absence or near absence of oxygen (this is a key concept in understanding backdraft and smoke explosion). The speed at which pyrolysis occurs is dependent on temperature and the rate of heat transfer to the fuel. When ordinary building contents or combustible structural materials undergo pyrolysis, are heated to their ignition temperature, and there is an adequate oxygen concentration, flaming combustion occurs.
One simple example of surface (heterogeneous) combustion occurs in a charcoal grill. Charcoal is comprised of carbon and (non-combustible) ash. When heated sufficiently, the carbon reacts with atmospheric oxygen at the surface level, releasing thermal energy. As an element, carbon cannot be decomposed and converted to the gas phase through pyrolysis, and as such there are no flames. Smoldering is another form of surface combustion that occurs at lower temperatures and can occur at lower oxygen concentrations than flaming combustion.
The fire triangle provides a simple (but not entirely accurate) model of combustion. Both flaming and surface (heterogeneous) combustion require heat, fuel, and oxygen. The fire tetrahedron is a better (but still not entirely accurate) model of flaming combustion with the addition of the uninhibited chemical chain reaction that results in flames.

Fuel
In a structural firefighting context, fuel is comprised of combustible building contents and structural materials. Fuel’s state of matter (solid, liquid, or gas), chemical makeup and configuration are important considerations when thinking about combustion and fire development. For now let’s focus on solid fuels typically comprising building contents and structural materials.
The chemical makeup of fuel determines its heat of combustion which is a measure of potential energy. Heat of combustion is a measure of how much energy is released when a specific amount of fuel is completely oxidized. Heat of combustion is expressed in joules per gram (J/g) or megajoules per kilogram (MJ/Kg). Alternately, heat of combustion could be expressed in British thermal units per pound (Btu/lb.).
The heat of combustion of synthetic, hydrocarbon-based fuels is considerably higher than that of wood products. For example, wood typically has a heat of combustion between 10 MJ/Kg and 15 MJ/Kg (Shi & Chew, 2021) while polyurethane foam used in furniture has a heat of combustion of approximately 27 MJ/Kg (Klausbruckner and Associates, 2014) and ABS plastic used in plastic shower enclosures has a heat of combustion of approximately 25 MJ/kg (D. Madrzykowski, personal communication, June 27, 2024). Many fuel packages are are comprised of multiple materials and have a heat of combustion based on the proportion of their various components.
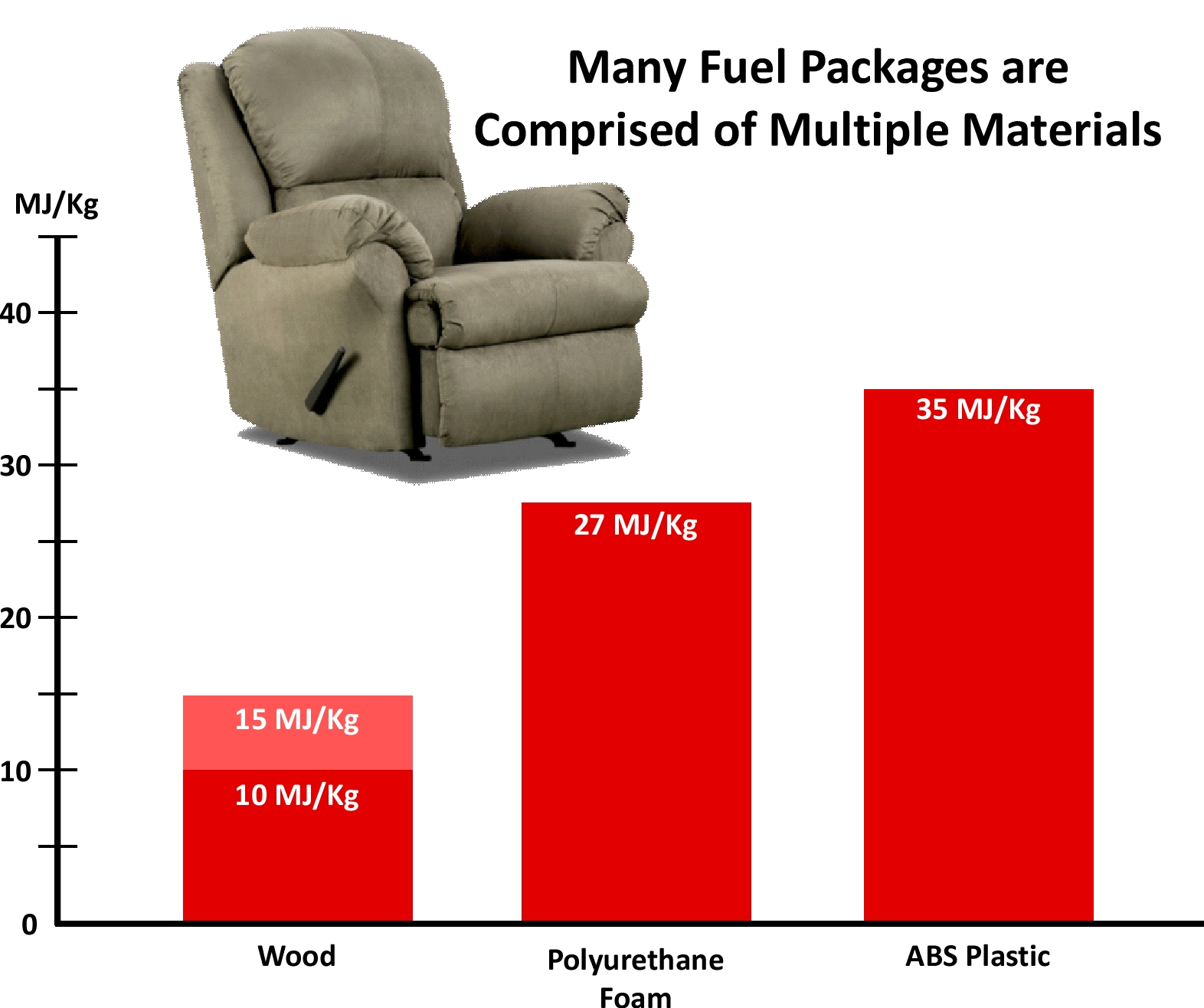
In addition to the potential energy of building contents and combustible structural materials, the speed with which they release energy is impacted by their surface to mass ratio and configuration. Heat release rate (HRR) is the fire’s power and is measured in Watts (W), Kilowatts (KW), or Megawatts (MW). A Watt is a Joule per second (J/s). In traditional US units of measure, heat release rate would be expressed in British thermal units per second (Btu/s). More on HRR in a bit.
Oxygen Consumption Principle
Release of chemical potential energy from fuel is dependent on availability of adequate oxygen for the combustion reaction to occur. Interestingly, while the heat of combustion of various types of organic (carbon based) fuel varies widely, the amount of oxygen required for release of a given amount of energy remains remarkably consistent.
The concept that energy release from an oxidation reaction such as a fire is related to the amount of oxygen consumed is not new. In 1866, James Braidwood wrote “The door should be kept shut while the water is being brought, and the air excluded as much as possible, as the fire burns exactly in proportion to the quantity of air which it receives” (Braidwood, 1866, p. 64). Fifty-one years later, William Thornton “discovered” that the energy released in an oxidation reaction is directly proportional to the mass of oxygen consumed in the reaction (Thornton,1917). Each kilogram of oxygen used in the combustion of common organic materials results in the release of approximately 13.1 MJ of energy. This is referred to as Thornton’s Rule.
Most firefighters have learned that the concentration of oxygen in the atmosphere is 21%. This is true in terms of volume. But if considered on the basis of mass, the concentration is somewhat different. Mass fraction is the mass of a specific substance in a mixture divided by the total mass of the mixture. The mass fraction of oxygen in air is 23.14% (The Engineering Toolbox, n.d.).
As Thornton’s rule is based on the mass of oxygen consumed, it is necessary to use the mass fraction of oxygen in air rather than the percentage by volume to calculate the energy that can be released by a kilogram of air. Multiplying 13.1 MJ/kg of oxygen by 23.15% gives a value of 3.03 MJ/kg of air. The Society of Fire Protection Engineering (SFPE) Handbook of Fire Protection Engineering rounds this value to 3.0 MJ/kg of air.
While it is easy to understand that air has mass, it is a bit more difficult to visualize a kilogram of air! The density of dry air at sea level and at a temperature of 20o C is 1.2 kg/m3 (0.075 lbs./ft3). Air density decreases as temperature or moisture increases, but this provides a starting point for visualizing the relationship between volume and mass at normal temperature and pressure.
If 1 m3 of dry air has a mass of 1.2 kg (2.64 lbs) multiplying 1.20 kg by 13.1 MJ/kg gives a value of 3.6 MJ (2,464.32 Btu). Illustrating that 1 m3 of air contains sufficient oxygen to release 3.6 MJ (2,464.32 Btu) of energy through combustion of organic materials.

With fires in buildings, combustion occurs in an enclosure where the air available for combustion is limited by:
- The volume of the compartment
- The extent to which ventilation provides for exchange of the atmosphere inside the compartment with that outside the compartment.
Heat
Heat is thermal energy in transit. In order for ignition to occur, thermal energy must be transferred to the fuel through convection, conduction, or radiation, raising its temperature to the point where it ignites (ignition temperature).
Sustained flaming combustion requires some thermal energy be transferred back to the burning fuel to continue pyrolysis, melting and vaporization or a combination of these processes. Thermal energy may also be transferred to additional fuel packages or other materials in the surrounding environment, allowing the fire to grow beyond the item first ignited.
Fire Development
When a fire has an ample supply of oxygen fire development is determined by the characteristics and availability of fuel including heat of combustion, surface to mass ratio, and arrangement. When these factors are the major influence on fire development, it is fuel limited. Without intervention, the fire progresses through the incipient, growth, and fully developed stages before decaying as the fuel is consumed.
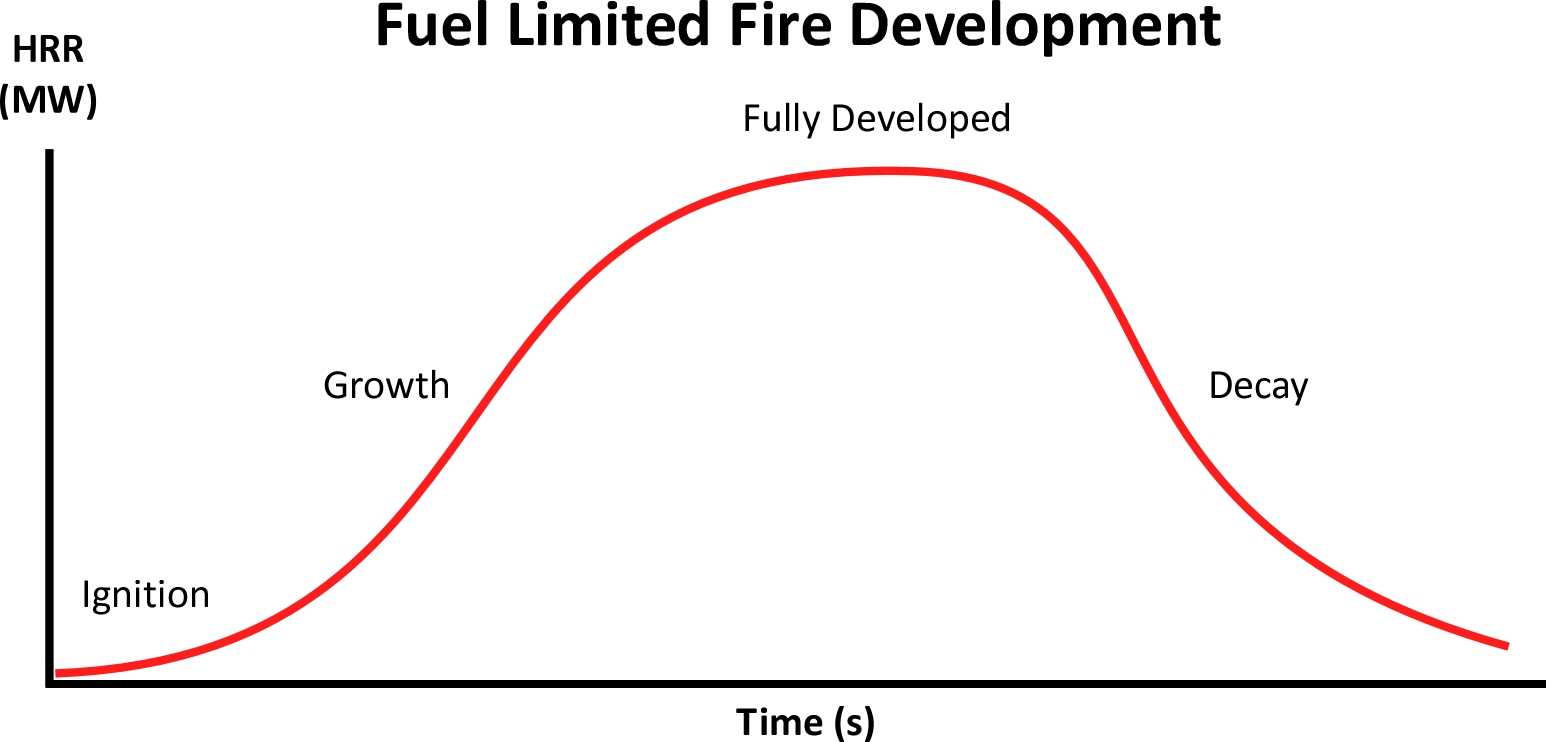
This fire development curve illustrates the life cycle of a fire that remains fuel controlled. When the fire occurs in an enclosure, fire development can follow a considerably different path.
When Do We Get to the Good Stuff?
So, just a couple of mentions of important concepts that tie into backdrafts and smoke explosions in this post. When do we get to the interesting bits? How do backdraft and smoke explosion conditions develop? What causes these phenomena? How do we recognize the potential for a backdraft or smoke explosion? And what can we do to prevent them (or can we)? Coming up in the next couple of posts!
References
Shi, L. & Chew, M. (2021) A review of thermal properties of timber and char at elevated temperatures. Indoor and Built Environment, 32(1). DOI 10.1177/1420326X211035557.
Thornton, W. (1917). The relation of oxygen to the heat of combustion of organic compounds, The London Edinburgh and Dublin Philosophical Magazine and Journal of Science, 33, 196-203.
Society of Fire Protection Engineers (SFPE) (2016). SFPE handbook of fire protection engineering. Gaithersburg, MD: Author.
The Engineering Toolbox (n.d.). Air – composition and molecular weight [webpage]. Retrieved April 8, 2025, from https://bit.ly/3EdvBvd.
Parker, W. (1977). An investigation of the fire environment in the ASTM E-84 tunnel test, NBS Technical Note 945. Gaithersburg, MD: National Bureau of Standards.
Klausbruckner and Associates. (2014). Fire Hazards Of Polyurethane Foam. Retrieved May 3, 2025, from https://bit.ly/4k0sTbJ.