Errata
Thanks to Dr. Stefan Svensson for identifying an issue in Backdraft and Smoke Explosion: Part 2. The term surface combustion was taken from the 14th Edition of the Fire Protection Handbook (NFPA, 1976). I had always thought that the explanation of flaming and “surface” combustion from this text was simple and easy to understand. However, within the context of fire dynamics, the current term for reaction of atmospheric oxygen and solid fuel on the fuel’s surface is called heterogeneous combustion. This differs from flaming or homogenous combustion where the reaction takes place between gas phase fuel and atmospheric oxygen where fuel and air are mixed prior to ignition. Backdraft and Smoke Explosion: Part 2 has been updated.
Enclosure Fires
The previous post in this series examined fuel-limited combustion in which there is no constraint on the availability of atmospheric oxygen. However, things become a bit different when a fire occurs in an enclosure such as a room or building.
Start with a simple example, a small room with a ceiling, floor, and four walls, a door on one side and a window on the opposite side and normal bedroom furnishings. If the window and door are closed, the air available to support combustion is limited to the volume of the room (and a little leakage from around the window and door). If the window or door is open, more air is available to support combustion, but air flow into the room will be limited by the size and position of the opening.
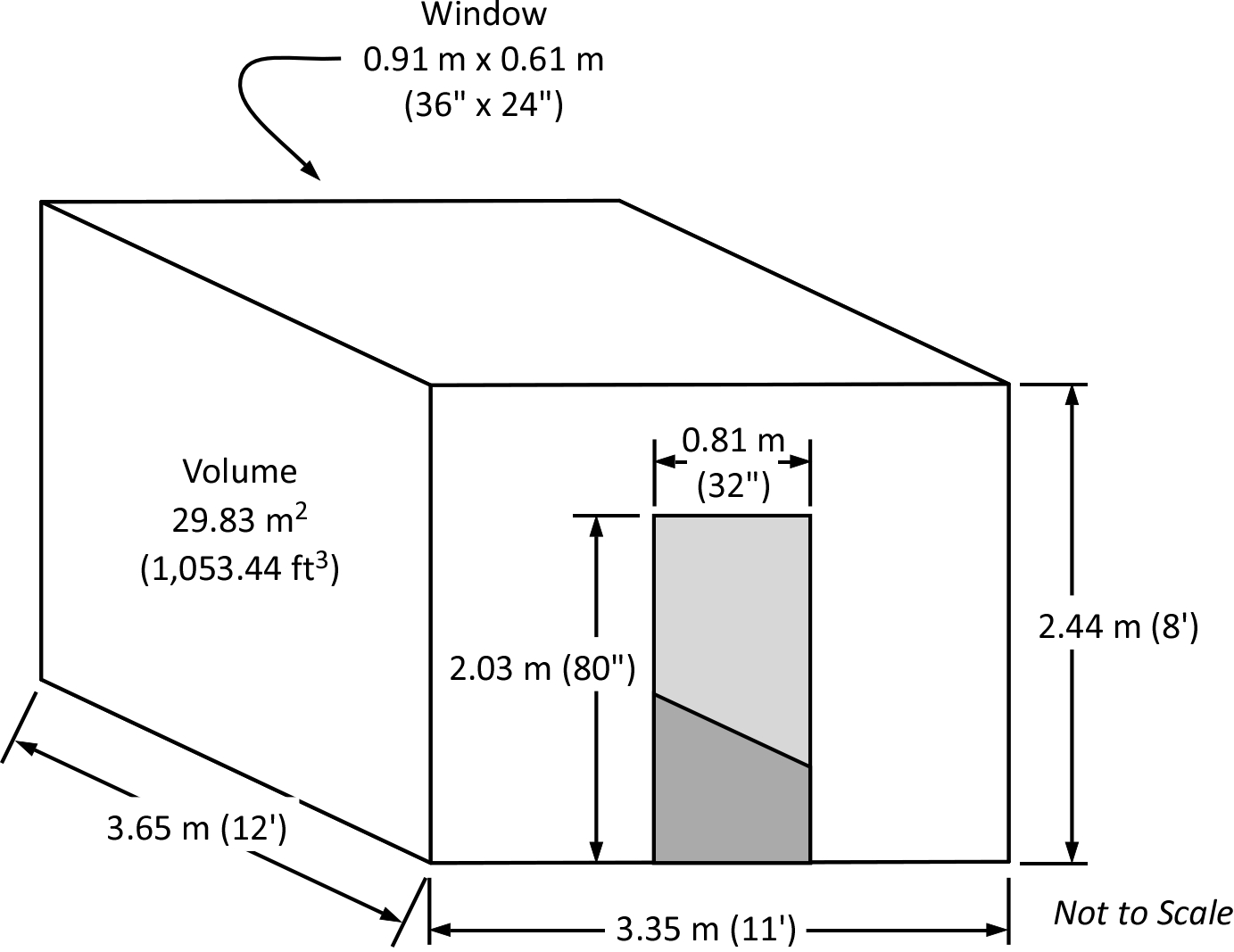
This room has a volume of 29.83 cubic meters (m3) of air not accounting for the volume of air displaced by the contents of the room. Put in terms of mass, the room contains 35.80 kg of air, which provides sufficient atmospheric oxygen to release 128.87 MJ of thermal energy (if all the atmospheric oxygen was consumed). If the door and window were closed, and the window didn’t fail, a fire burning with a heat release rate (HRR) of 1 megawatt (MW) would consume all the atmospheric oxen within this compartment in just over two minutes. While fires involving solid fuels such as furnishings and interior finish materials do not start out with a HRR of 1 MW, it is easy to see how a fire in this compartment could quickly become ventilation-limited and potentially self-extinguish.
However, if the window fails, the door is open, or both the window and door are open, the fire will likely progress quickly from the growth stage through flashover to a fully developed fire. Fully developed fires within an enclosure are ventilation-limited as increasing ventilation will increase the HRR.
Buildings can be a bit more complex, and may have multiple rooms, interior doors, windows and exterior doors. In addition, buildings generally have some amount of designed ventilation and may also be insulated to limit heat transfer through exterior walls. Compartmentation, designed ventilation, potential ventilation openings (think doors and windows), and insulation all have an impact on fire development.
Fire Development with Limited Ventilation
In an enclosure with limited volume and ventilation, fire development may take a different path. As fire develops, it consumes oxygen and as HRR increases, it consumes oxygen at a faster rate. With limited enclosure volume and ventilation, oxygen concentration decreases, and this soon becomes the determining factor in fire development. If there is sufficient fuel and atmospheric oxygen the compartment of origin may transition through flashover to a fully developed fire. If the compartment of origin is small and has limited ventilation (e.g., closed door), the fire may not transition through flashover to a fully developed stage. In a ventilation-limited burning regime, increasing HRR may slow, peak at a lower level, and as oxygen concentration continues to decrease so too will HRR and temperature. The fire will begin to decay (Bengtsson, 2001). If the oxygen concentration falls below 13%-15%, flaming combustion will cease (Quintiere, 2016) but pyrolysis and heterogenous combustion will continue.
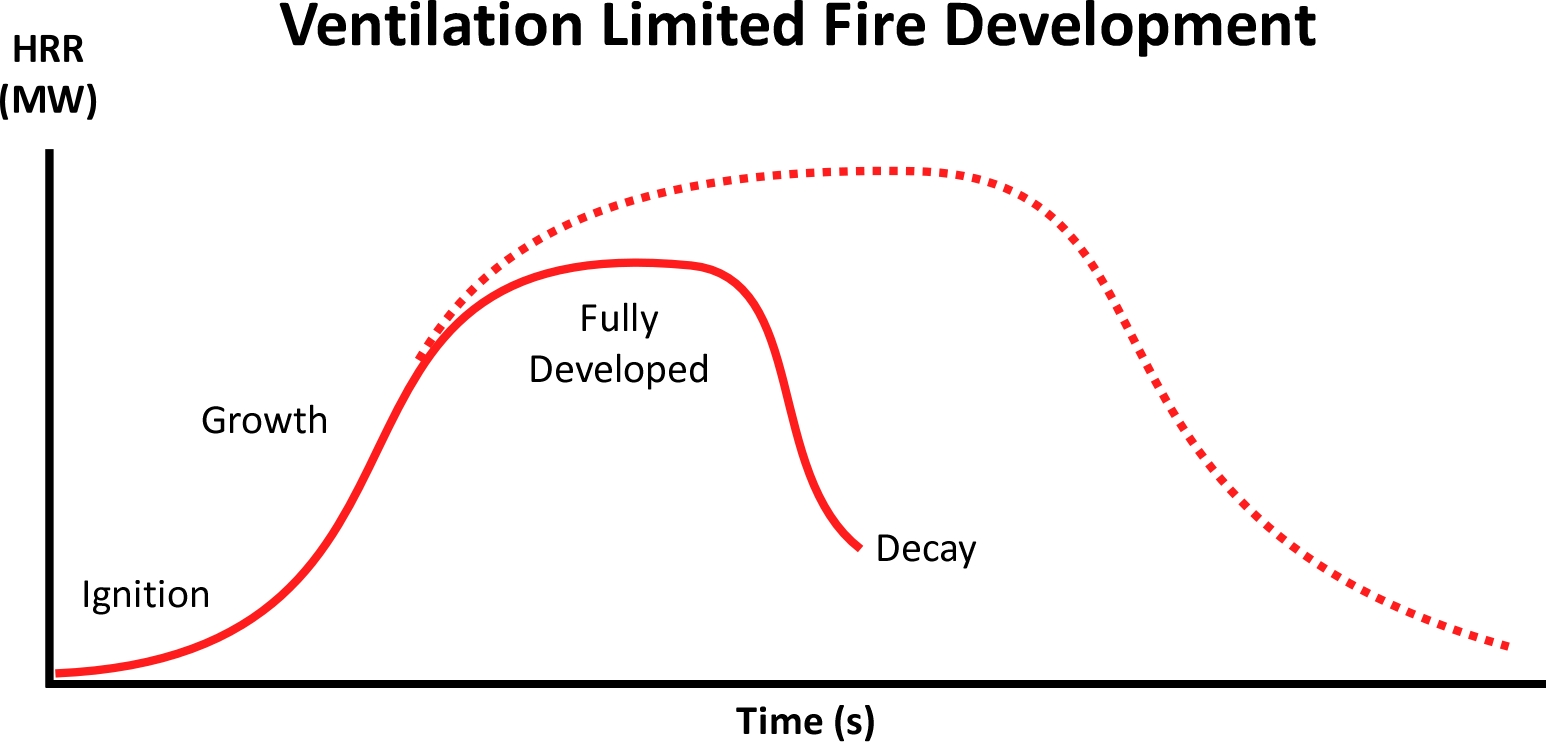
The dotted line represents the potential HRR if the fire was not limited by the available atmospheric oxygen. The actual HRR curve can vary considerably depending on the fuel type and ambient ventilation of the enclosure. However, in typical residential or commercial occupancies the fire will become ventilation-limited at some point in its progression.
When a fire is in the growth stage, the HRR and temperature within an enclosure will increase. Increased temperature causes the air and fire gases within the space to expand, becoming more buoyant and will increase pressure within the enclosure. The buoyancy and pressure resulting from increased temperature causes smoke to push out of leakage points (e.g., around windows and doors, attic vents, etc.).
Normal building ventilation and leakage may not be sufficient to maintain continued fire growth. As the ventilation-limited fire enters the decay stage, the HRR and resulting temperatures will become lower, reducing pressure within the enclosure. As pressure drops, the exhaust of smoke through leakage points will become less pronounced and may cease entirely as the leakage points become air inlets. Intake of air will result in regrowth of the fire with increasing HRR and temperature, returning the leakage points to exhaust openings for smoke. The increased HRR results in increased oxygen consumption, resulting in the cycle repeating itself (Lambert & Baaij, 2011).

With pulsating fire development, there is sufficient temperature is maintained for ongoing pyrolysis of building contents and structural materials, increasing the concentration of flammable pyrolysis products in the smoke.
There is a significant difference between decay as fuel is consumed and decay resulting from oxygen deficiency. Decay due to consumption of fuel is irreversible while decay due to reduced oxygen concentration will be reversed, and the fire will return to the growth stage if ventilation is increased prior to the fire self-extinguishing due to a lack of oxygen.
As a fire in an enclosure becomes ventilation-limited, combustion is less efficient and smoke production increases. Some products of incomplete combustion are flammable (like carbon monoxide (CO)) and thermal energy released by the fire may also result in production of more (flammable) pyrolysis products than can be consumed in the combustion reaction.
Smoke
Smoke is an aerosol comprised of air, and the gases, vapors, and particulate matter produced by the fire. Products of combustion from a fire occurring in an enclosure include (but are not limited to) carbon dioxide, carbon monoxide, nitrogen oxides, hydrogen sulfide, water vapor and hydrogen cyanide, heavy hydrocarbon liquid particles, and soot (solid carbon particulate). The exact composition of fire gases is determined by the nature of the fuel that is burning, the concentration of air available for combustion, and the temperature within the fire area.
Dr. Stefan Svensson (2020) identifies that there are five aspects of fire gases that should be considered.
- Fire gases can have low transparency, thus limiting vision.
- Fire gases can be combustible, even if they are completely transparent.
- Fire gases are generally toxic and affect the body in both the short and the long term.
- Fire gases can be reactive and can then affect and destroy interiors, tools, or other equipment.
- Fire gases may be hot (p. 34).
In the context of examining backdrafts and smoke explosions, two of these aspects are particularly important; that fire gases can be combustible and that they may be hot.
Just as the products of incomplete combustion can vary with conditions, the extent to which the mixture of products of combustion is flammable is also variable. Flammability is based on what products are present, their concentration as a percentage of the total mass of air and fire gases, mixing of the gases, their temperature, and the presence of thermal ballast (products which do not participate in the combustion reaction, but must be heated along with the fuel and air). Smoke is fuel!
Firefighters are generally familiar with the concepts of the lower flammable or explosive limit (LFL or LEL), upper flammable or explosive limit (UFL or UEL), and flammability range for flammable gases and vapors such as methane, propane, and gasoline vapor.
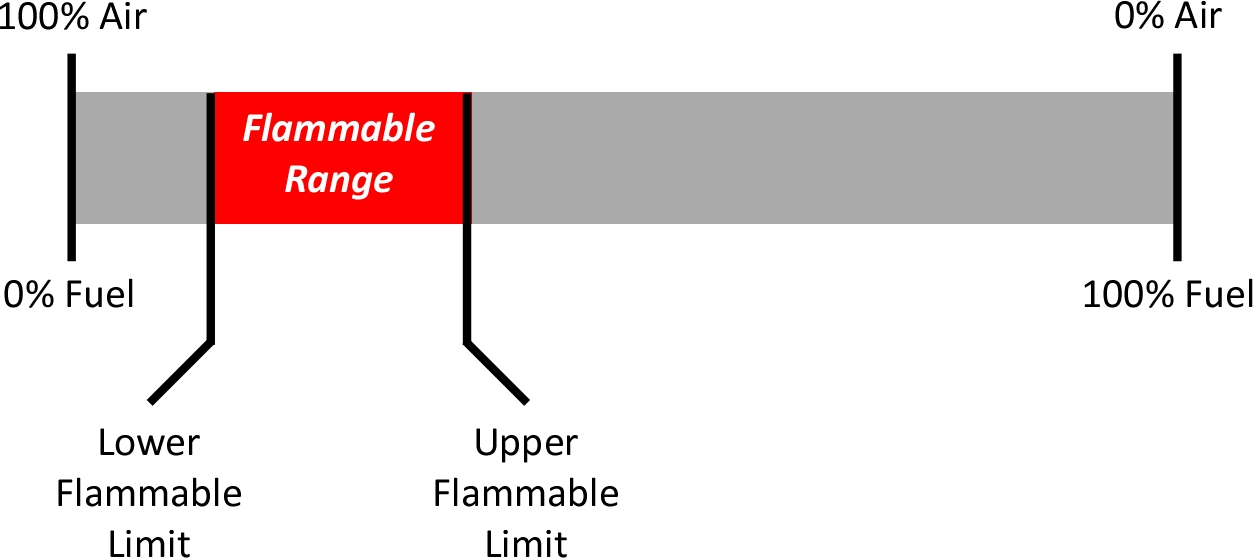
This simple look at flammable range is dependent on air containing approximately 21% oxygen by volume. However, fuel gases and vapors can burn in atmospheres with varying concentrations of fuel, oxygen, and inert gases and passive agents not participating in the combustion reaction (e.g., nitrogen). A Shapiro-Maffetti diagram or flammability diagram illustrates the flammability of gas mixtures. This diagram is a triangular graph used to represent the concentration of fuel, oxygen, and inert gases and passive agents where their proportions always total to a value of 100%. The diagram helps in understanding the conditions under which a mixture can ignite and burn. The flammability diagram of methane (Wilson & Mortimer, 2020) is used as an example.
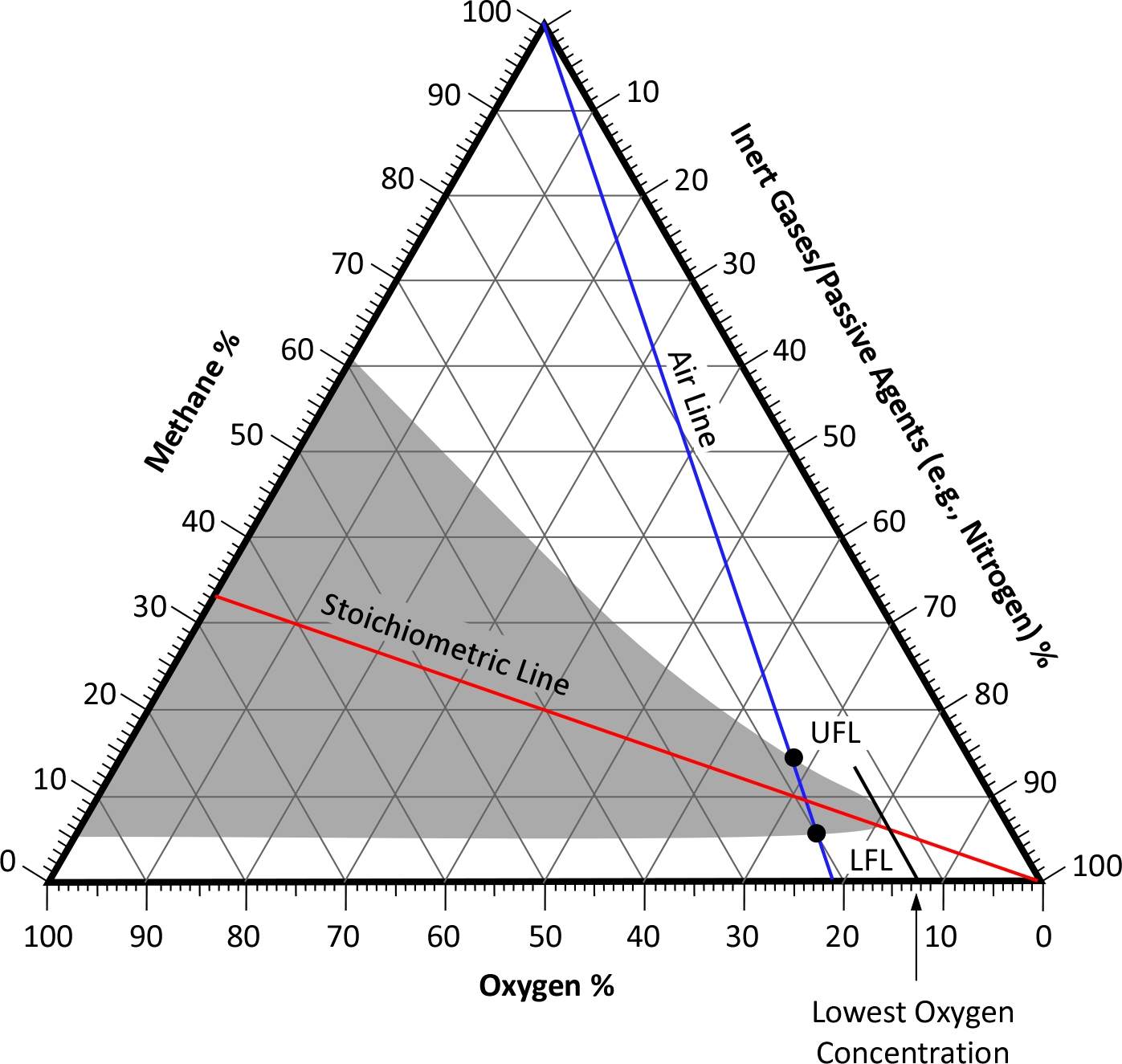
In this diagram the shaded area indicates the range of conductions under which methane is flammable. The blue line and points labeled UFL and LFL tie directly to the simpler diagram of upper and lower flammable limits and flammable range. But as the flammability diagram illustrates, methane will burn at oxygen concentrations down to approximately 12% by volume. The red Stochiometric Line illustrates the ideal mixture(s) that will result in complete (and most rapid) combustion. The diagrams for other flammable gases and vapors will be similar, but not identical and flammability diagrams can be developed for known mixtures of fuel gases. The problem with the concentration of fuel, oxygen, and inert gases and passive agent concentration within an enclosure under fire conditions is that the exact mixture is unknown and will vary in different locations within the enclosure and from one point in time to another
Movement of smoke within an enclosure, from compartment to compartment, and into structural void spaces is influenced by buoyancy and pressure. As the temperature of air and fire gases increase due to release of thermal energy by the fire they will expand, becoming less dense and more buoyant, and will rise. Reaching the ceiling, smoke will begin to travel horizontally, cooling somewhat as it moves away from the fire. When the expansion of air and fire gases is constrained by the volume of the enclosure and limited exhaust openings, pressure will increase. Smoke will move from areas of high pressure to those with lower pressure.
Dr. Stefan Svensson (2020) identifies six factors that influence the flow of fire gases within buildings:
- Thermal buoyancy (differences in density).
- Thermal expansion.
- The amount of products of combustion produced by the fire.
- Temperature differences between indoor and outdoor air.
- External wind conditions.
- Heating, ventilation, and air conditioning (HVAC) systems and other normal building ventilation (p. 42)
It is important to understand the movement of smoke and the potential variation in the concentration of smoke (fuel), atmospheric oxygen, and inert gases and passive agents within various compartments and void spaces within the building.
Up Next
Next week we will dig into the backdraft phenomenon and take a look at the differences between ventilation induced flashover and backdraft (as both result from an increase in ventilation), the conditions necessary for a backdraft to occur, and potential signs of backdraft potential.
References
Bengtsson, L. (2001). Enclosure fires. Karlstad, Sweden: Räddnings Verket.
Lambert, K. & Baaij, S. (2011). Brandverloop: technisch bekeken, tactisch toegepast. Rotterdam, Netherlands: Sdu Klantenservice.
Quintiere, J. G. (2016). Compartment Fire Modeling. In M. J. Hurley (Ed.), SFPE Handbook of Fire Protection Engineering, 5th ed., 1062–1104). Springer. https://doi.org/10.1007/978-1-4939-2565-0.
Svensson, S. (2020). Fire ventilation. Retrieved May 11, 2025, from https://bit.ly/3W3nJm0.
Wilson, S. & Mortimer, S. (2020). Geological Society, London, Engineering Geology Special Publications, 29, 457-478. DOI: https://doi.org/10.1144/EGSP29.19.