Backdraft Preconditions
Avoiding adverse outcomes from the occurrence of backdrafts requires a sound understanding of this fire behavior phenomena and the conditions under which a backdraft may occur.
Engaging with the task of distilling backdraft scientific research into an accurate, but practical explanation for operational firefighters and fire officers and sorting through backdraft indicators identified through scientific research and operational experience has proved to be an interesting, but challenging experience.
Former United States Defense Secretary Donald Rumsfeld divided information into a matrix with four categories, known knowns, known unknowns, unknown knowns, and unknown unknowns. Most firefighters and officers have some knowledge of what a backdraft is and how this phenomena occurs. However, backdraft is a complicated phenomenon, and it is quite a task to develop a solid working understanding of the prerequisites and influences on the occurrence of a backdraft. Think about what you know and what you don’t know!
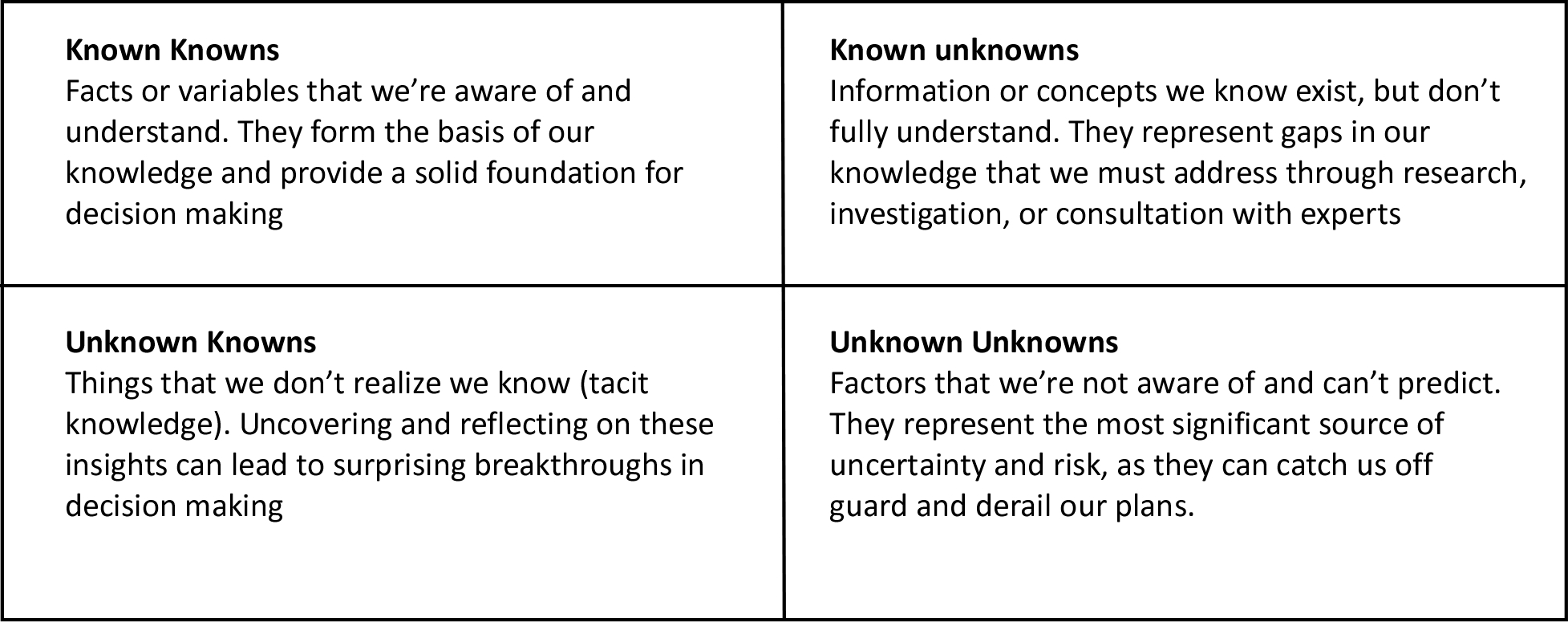
A quote attributed to author Mark Twain provides an important warning; “It ain’t what you don’t know that gets you into trouble. It’s what you know for sure that just ain’t so”. Knowledge is not static; it is ever evolving. We often hold onto what we first learned, and we may be reluctant to challenge what we think we know, but this is an important part of developing professional expertise!
Backdraft Research
The first known article discussing backdraft as “ignition of mixtures of air with the minute particles of unconsumed carbon and invisible gaseous matter in smoke from the imperfect combustion of organic substances” (Steward, 1914, p. 427). Steward also used the terms smoke explosion, back draught, and hot air explosion to describe the same phenomena.
In 1975, a “backdraft” in a mattress store in Kent, England resulted in the death of two firefighters resulting in an extensive literature review examining fires involving explosions (Croft, 1980 as cited in Fleischmann, 1994). This incident also prompted a research project examining the explosive risk of foamed rubber by the British Building Fire Research Establishment (Wolley & Ames, 1975). Fleischmann (1994) identified that this was likely the first experimental research investigating the backdraft phenomena. Wolley and Ames performed qualitative, bench scale tests on flaming and smoldering combustion of foam rubber like that in the Kent incident as well as small scale (1.41 m3) tests where combustion and pyrolysis products from smoldering foam rubber were ignited by a pilot flame. However, there was no change in ventilation and the oxygen concentration within the chamber was reported to be 19.25% by volume. Given the test conditions the resulting explosion would be more appropriately classified as a smoke explosion (rather than a backdraft). More on this in a subsequent post!
Between 1975 and 1994, there was little research and little progress in developing an understanding of the backdraft phenomena. In 1994, the National Institute for Standard and Technology (NIST) published Backdraft Phenomena (Fleischman, 1995). This was the first substantive examination of the backdraft phenomena and provided a foundation for ongoing research.
Since 1994, there have been multiple studies investigating factors that influence the occurrence of backdrafts. As with many other avenues of scientific inquiry, each study provides answers within the context of the experimental design. This requires limiting the number of variables that are manipulated so that the data allows inference of what factors influence backdraft occurrence.
Most backdraft research is conducted in a single compartment, often on a reduced scale due to the significant expense and logistical challenges with conducting full-scale experiments. Bolliger (1995) investigated the influence of scale on backdraft experimental research and determined that “comparison of results between those obtained during this research and those obtained from Fleischmann (1993) compare well” (p. 77) and as such, “scale has no obvious effect on backdraft” (p. xi).
Backdraft experiments have been conducted using gas, liquid, and solid fuels. The following table identifies some of the backdraft research conducted with different types of fuel and at differing scales.
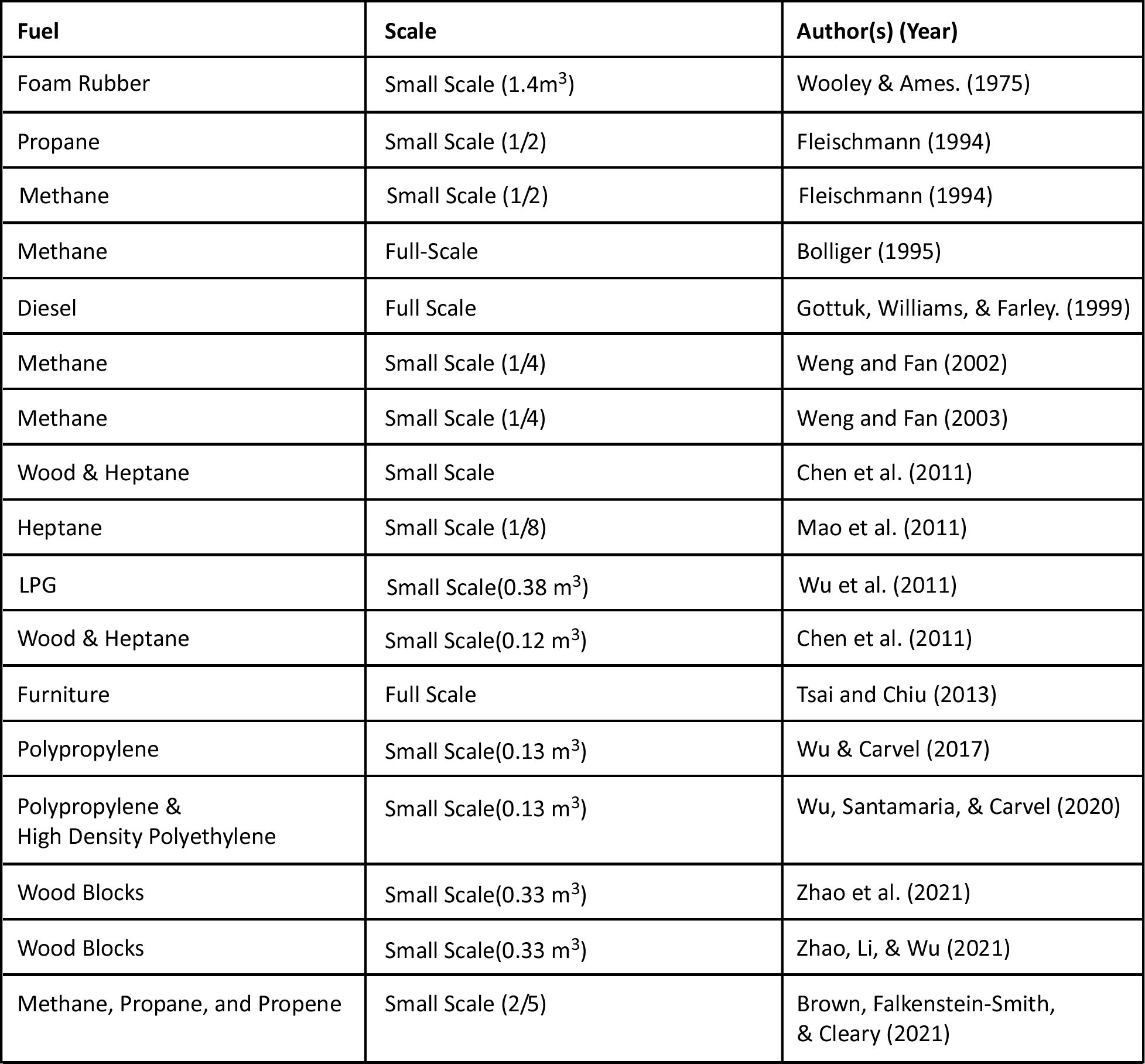
As can be seen in the preceding table, most of the backdraft research has used flammable gases (e.g., methane or propane) or flammable liquids (e.g., diesel or heptane). Other experiments have used thermoplastics (e.g., polypropylene or high density polyethylene). In contrast, backdraft events in the built environment “occur in compartments filled with complex mixtures of partially burnt products of pyrolysis from solid fuels such as wood, plastics and foams. Such pyrolysis gases have a range of flammability limits and may only be flammable at elevated temperatures” (Wu, Santamaria, & Carvel, 202, p.2).
Factors Influencing Occurrence of a Backdraft
Examining research conducted on the backdraft phenomena since Fleischmann’s Pagni’s & Williamson’s initial work in1993, several common factors can be identified.
- There must be a high mass fraction of flammable products of pyrolysis and combustion within the enclosure.
- The mass fraction of flammable products of pyrolysis and combustion must be above the flammable range of the mixture, given the mass fraction of oxygen and temperature within the enclosure.
- The greater the mass fraction of flammable products of pyrolysis and combustion, the more severe the backdraft will be and the greater the pressure that will potentially be developed.
- The mass fraction of oxygen will be lower than normal due to combustion within an enclosure with limited ventilation.
- A ventilation opening must be created in a location that will allow exhaust of hot, less dense products of pyrolysis and combustion and intake of cool, fresh air resulting in creation of a gravity current resulting in mixing of air and fuel rich smoke, creating a flammable mixture.
- The products of combustion and pyrolysis must be at an elevated temperature but not necessarily above the autoignition temperature of the mixture for a backdraft to occur. This temperature is specific to the fuel.
- If the gas temperature or compartment boundaries (linings) are above the ignition temperature of the mixture of products of combustion and pyrolysis, they may auto-ignite following an increase in ventilation and increase in the mass fraction of oxygen within the enclosure.
- If fire involves fuels that are subject to heterogeneous (smoldering, surface) combustion (e.g., solid fuels such as wood), increased ventilation may increase the heat release rate of smoldering fuels sufficiently to provide piloted ignition of the products of pyrolysis and combustion.
- If backdraft conditions exist (as outlined above) there will be a delay between creation of a ventilation opening and occurrence of the backdraft due to the transit time of the gravity current through the enclosure.
Back to the Definition of Backdraft
Part 1 of this series of blog posts identified that both NFPA 921 and NFPA 1700 define backdraft as “a deflagration resulting from the sudden introduction of air into a confined space containing oxygen-deficient products of incomplete combustion” (NFPA, 2024, 3.3.19 & NFPA, 2022, 3.3.6) and asked what is missing? “
The earliest speculation regarding the nature of backdrafts focused on carbon particulate as the fuel contributing to a backdraft (Steward, 1914). Since that time, there has been a commonly held belief that carbon monoxide was the major fuel constituent in a backdraft (Casey, 1968; Clark, 1974). However, the lower explosive limit of carbon monoxide is 12.5% and the upper explosive limit is 74%, neither of which are commonly reached in the fire environment. The missing element in this definition is pyrolysis as unburned pyrolizates which are the predominant fuel for backdrafts (Fleischmann, 1004).
Concentration of Gas Phase Fuel
Fleischmann’s early research on backdrafts using a small-scale compartment, a methane fueled fire, and piloted ignition (electrical igniter) indicated that unburned mass fractions (percentage by mass) of greater than 10% were required for the occurrence of a backdraft (Fleischmann, 1993). Fleischmann also observed that “hydrocarbon concentrations > 15% exhibit large fire balls outside the compartment and would result in significantly higher pressures if contained within an adjacent corridor or room with a volume of the order of the experimental compartment”. Other research affirmed the importance of the mass fraction of unburned flammable products of pyrolysis and combustion and that the dominant fuel in a backdraft is flammable pyrolizates (Bolliger, 1995; Gottuk, Williams, & Farley, 1999; Gojkovic, 2001; Weng & Fan, 2002, 2003; & Mao et al., 2011). However, the minimum mass fraction of fuel for the occurrence of a backdraft ranged from 7% Weng & Fan (2003) to 15% (Bolliger, 1995). Backdrafts in the built environment are fueled by pyrolysis products from a wide range of natural (e.g., wood) and synthetic fuels (e.g., plastics) which likely have somewhat different flammability characteristics than methane.
Gottuk, Williams, & Farley (1999) demonstrated that a flammability diagram can be used to predict the bounding limits and trends related to the impact of the mass fractions of oxygen, fuel, and nitrogen (as a proxy for all non-flammable constituents within the compartment.

Note: Adapted from Gottuk, D.; Williams, F.; & Farley, J. (1997). The development and mitigation of backdrafts: a full-scale experimental study. Fire Safety Science, 5, 935-936. doi: 10.3801/IAFSS.FSS.5-935.
The first example considers conditions where all atmospheric oxygen has been consumed leaving a mixture of fuel and nitrogen until the door to the compartment is opened.
- Point A is the percentage by mass of normal oxygen concentration in air (23.14%).
- Point B is a mixture of 5.00% fuel and 95.00% nitrogen.
- Point C represents an initial mixture of 11.0% fuel and 89.00% nitrogen
- Point D represents an initial mixture of approximately 17.50% fuel and 82.50% nitrogen.
The line connecting Points B and A illustrates the mixtures that may be created if a door to the compartment is opened allowing air to enter and mix with 5% fuel and 95% nitrogen. As this line does not intersect the flammability envelope, a flammable mixture will not result, and an explosion will not occur.
The line connecting Points C and A illustrates the mixtures that may be created if a door to the compartment is opened allowing air to enter and mix with 11% fuel and 89% nitrogen. As this line is tangent to the flammability envelope, it represents the minimum fuel mass fraction necessary to obtain a flammable mixture once mixed with air.
Any mixture of fuel and nitrogen with a fuel percentage by mass of greater than 11% will result in a flammable mixture when mixed with sufficient air. This is represented by the line connecting Points D and A which intersects the flammability envelope.
This scenario, based on complete consumption of the atmospheric oxygen in the compartment is closely representative of the backdraft study by Gottuk, Williams, and Farley (1997).
However, in buildings, it is quite likely that the oxygen concentration will be low enough for flaming combustion to cease, but not reach zero due to normal ventilation (e.g., heating, ventilation, and air conditioning (HVAC) systems and leakage).
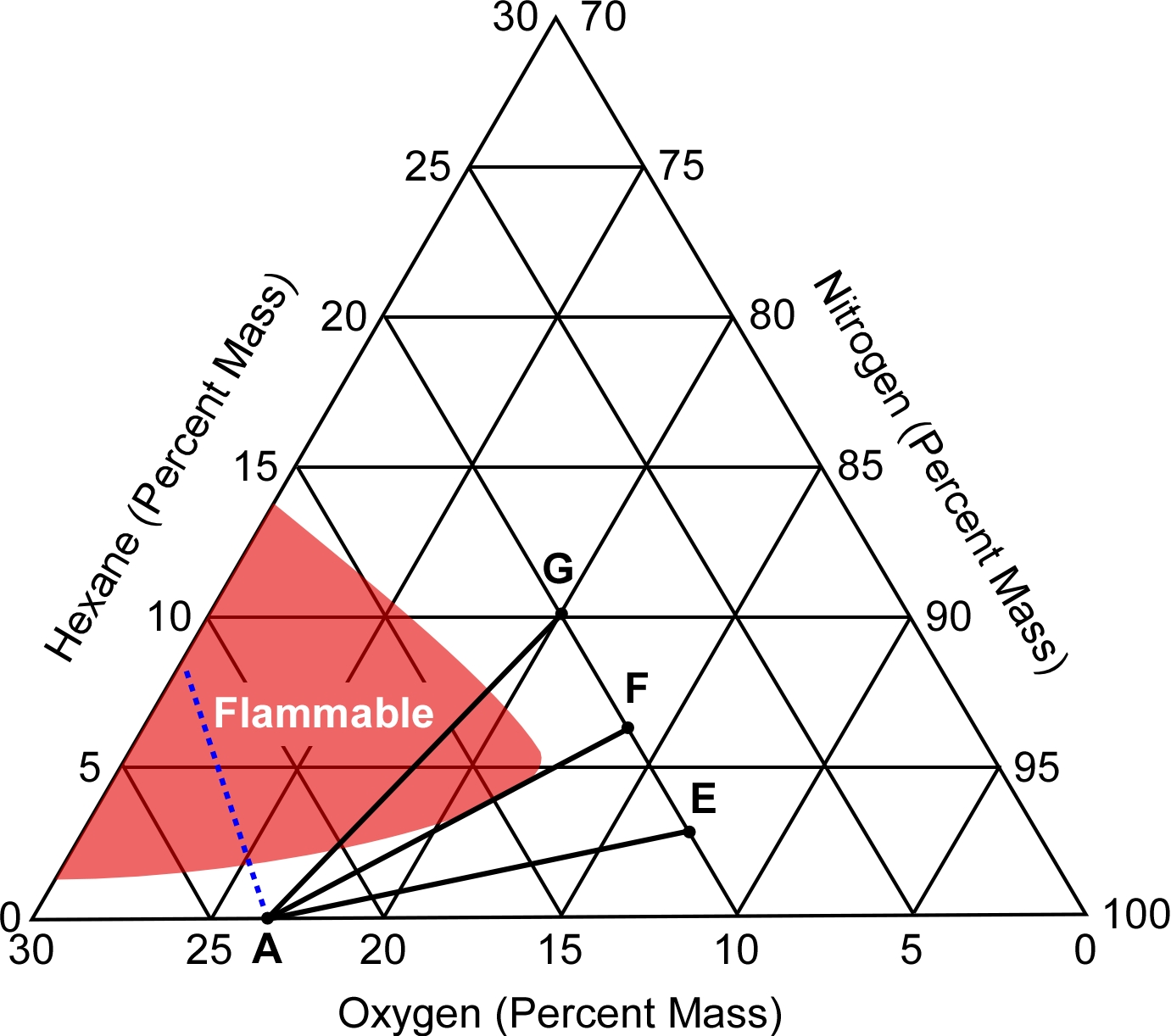
Note: Adapted from Gottuk, D.; Williams, F.; & Farley, J. (1997). The development and mitigation of backdrafts: a full-scale experimental study. Fire Safety Science, 5, 935-936. doi: 10.3801/IAFSS.FSS.5-935.
Gottuk, Williams, and Farley (1997) also illustrate these conditions using the flammability diagram.
- Point A is the percentage by mass of normal oxygen concentration in air (23.14%).
- Point E is a mixture of 3.00% fuel and 87.00% nitrogen and 10.00% oxygen.
- Point F is a mixture of 6.5% fuel and 83.50% nitrogen, and 10.00% oxygen
- Point G is a mixture of 10.00% fuel and 80.00% nitrogen and 10.00% oxygen.
The line connecting Points E and A illustrates the mixtures that may be created if a door to the compartment is opened allowing air to enter and mix with 3.00% fuel and 87.00% nitrogen and 10.00% oxygen. As this line does not intersect the flammability envelope, a flammable mixture will not result, and an explosion will not occur.
The line connecting Points F and A illustrates the mixtures that may be created if a door to the compartment is opened allowing air to enter and mix with 6.5% fuel and 83.50% nitrogen, and 10.00% oxygen. As this line is tangent to the flammability envelope, it represents the minimum fuel mass fraction necessary to obtain a flammable mixture once mixed with air.
Any mixture of fuel and nitrogen with a fuel percentage by mass of greater than 6.5% will result in a flammable mixture when mixed with sufficient air. This is represented by the line connecting Points G and A which intersects the flammability envelope.
This example shows how the critical fuel mass fraction for backdraft conditions decreases with increasing oxygen concentration. The critical fuel mass fractions at oxygen concentrations of 0% and 12% percent (typical oxygen limit for flaming combustion of most hydrocarbon fuels) represent bounding values (Gottuk, Williams, and Farley, 1997)
One caveat with the use of flammability diagrams is that flammable range is influenced by temperature. As the temperature increases, so does flammable range. As illustrated below, a mixture may not be flammable at a given temperature (T1) but if the temperature of the mixture increases it may become flammable (T2).
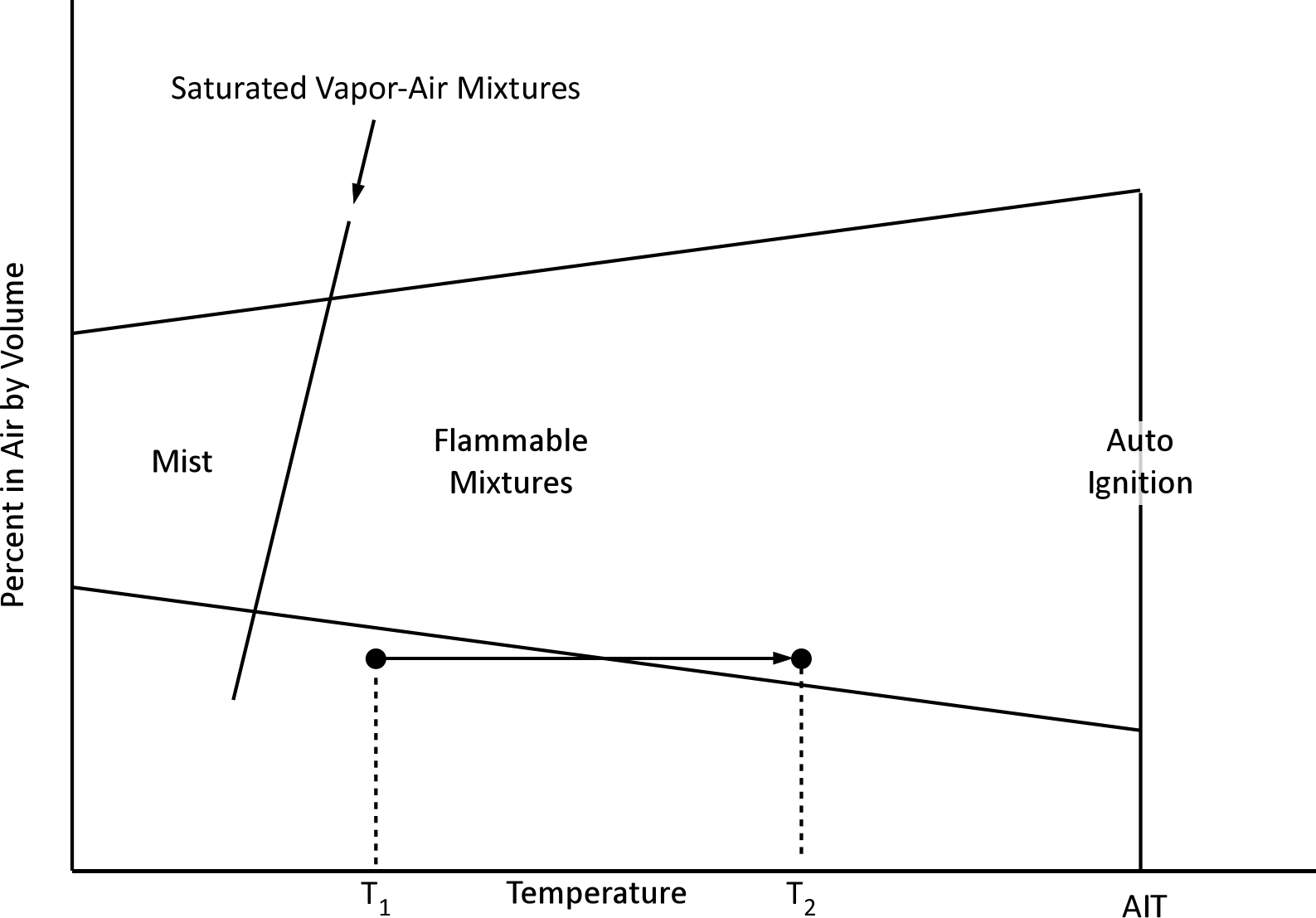
Note: Adapted from Zabetakis, M. (1965). Flammability characteristics of combustible gases and vapors. Washington, DC: United States Department of the Interior, Bureau of Mines. https://bit.ly/4mSK01F.
“Real backdraft incidents involve complex fuel gas mixtures consisting of the products of underventilated burning and pyrolysis… However, most experimental research into backdraft has used methane gas or flammable liquids as fuel. Some aspects of real backdraft behavior may have been overlooked as a consequence of this simplicity” (Wu, Santamaria, & Carvel, 2020, p. 937).
The important takeaway is that a high concentration flammable products of pyrolysis and combustion are required and that a minimum concentration is necessary for a backdraft to occur, but the minimum concentration likely varies based on the flammable products of pyrolysis and combustion involved.
Gravity Current
In fluid dynamics, a gravity current or density current is a primarily horizontal flow in a gravitational field that is driven by difference in the density of a fluid or fluids and is constrained to flow horizontally. In an enclosure filled with hot smoke, the fluids are the hot smoke and cooler outside air, and the constraint is the ceiling of the enclosure.
When a fire occurs in an enclosure where the only ventilation is through small vents and construction gaps, it will continue to grow based on the available fuel until it becomes ventilation limited. During the fire’s growth stage, a hot layer will form at the ceiling level and then will descend as smoke fills the enclosure. The fire will consume oxygen within the enclosure, leading to more incomplete combustion, and accumulation of unburnt pyrolizates in the hot upper layer. As the oxygen concentration continues to decrease the fire will begin to decay and the concentration of flammable products of pyrolysis and combustion will increase. When the oxygen concentration becomes low enough, flaming (homogenous) combustion will cease and the fire may continue to smolder (heterogeneous combustion), or it may self-extinguish depending on the fuel supply and ventilation. As long as the temperature remains high enough, fuel within the enclosure will continue to pyrolyze, adding additional fuel into the smoke layer. While the temperatures in the enclosure after the fire has begun to decay are less than if the fire was fully developed (post flashover), they are still considerably above ambient temperature, making the smoke layer less dense (more buoyant) than the ambient temperature air outside the enclosure (Chitty, 1994; Fleischmann, 1994; & Bolliger, 1995)
If a door or window is opened or fails, creating a ventilation opening, hot buoyant gases will exit the enclosure from the top of the vent and cooler fresh air will enter at the bottom of vent creating a bi-directional flow. The inflow of cooler, more dense fresh air is the gravity current which progresses through the enclosure mixing with the fuel rich, oxygen deficient upper layer. This turbulent mixing along the top of the gravity current will be increased by flow across obstructions (e.g., furniture) and velocity of the gravity current which is impacted by the temperature difference between the layers (and potentially by wind). Mixing of the fuel rich upper layer and the cooler air in the gravity current result is creation of a flammable mixture at the interface of the two layers. If this flammable mixture is ignited within the enclosure, it may (depending on the mass fraction of fuel) result in a backdraft.
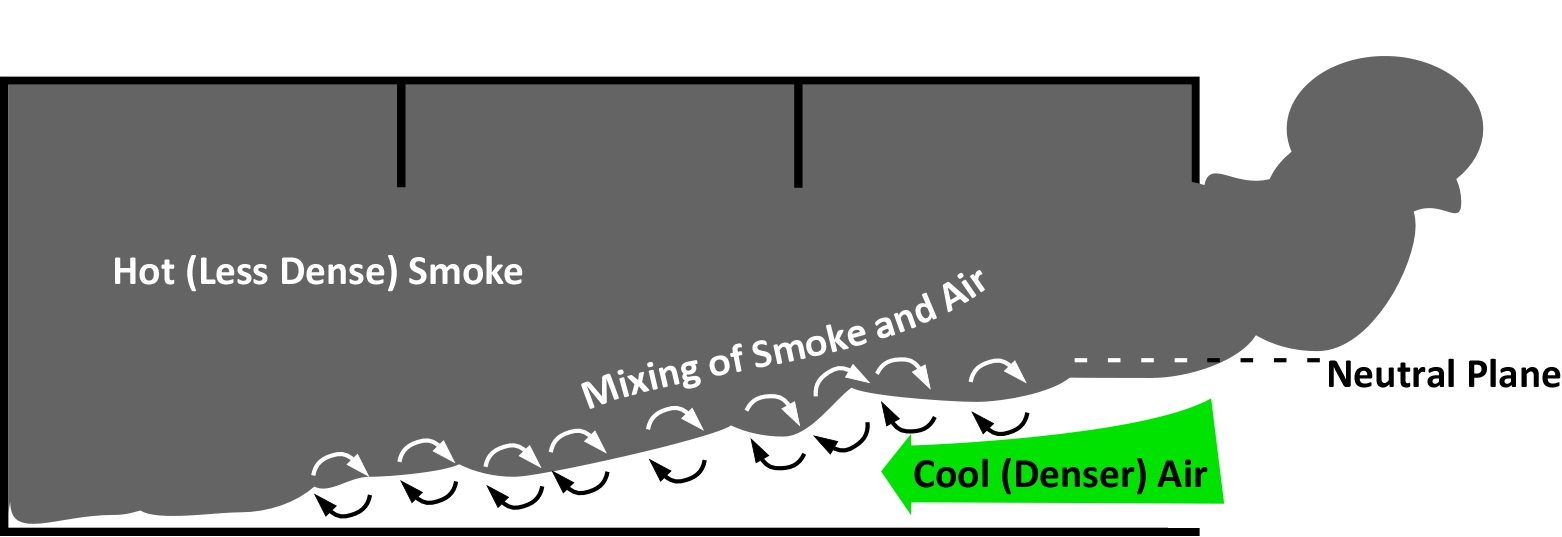
The mixing of hot, fuel rich smoke and cooler air is not instantaneous as it takes time for the gravity current to progress through the enclosure. Several researchers found that the location, configuration and size of the ventilation opening had an influence on the timing and severity of backdraft occurrences.
Wu et al. (2011) reported that it was easier to create a backdraft when the ventilation opening was smaller (with the ignition source placed further from the ventilation opening and the fuel concentration was higher). Zhao, Li, and Wu (2021) identified that when the ventilation opening was larger, the time for a backdraft to occur decreased. Zhao et al. (2021) reported similar results and identified that larger openings result in a more rapid increase in oxygen concentration. These varied observations regarding ventilation and its likely impact on the gravity current and mixing of air and fuel indicate that the opening is an important factor, but that additional research is likely necessary to develop an improved understanding of its impact.
Temperature
Temperature influences the process of pyrolysis. Pyrolysis is a thermochemical process of breaking fuel down into smaller molecules. This process does not require reaction with oxygen (this comes later in the combustion process). Consider the example of pyrolysis of wood; heating of wood first drives off moisture and then cellulose, hemicellulose, and lignin is broken down into smaller molecules. Pyrolysis is endothermic, meaning it requires heat input to proceed. Heat breaks the chemical bonds in the wood, releasing gases like methane, carbon monoxide, and hydrogen, along with liquid tar and solid char. Pyrolysis of wood begins at temperatures ranging from 200–300 °C (390–570 °F) with a faster rate of heating yielding a larger amount of volatile gases (Kintek, 2025). The pyrolysis of wood is only one example. As illustrated below, the process of pyrolysis varies considerably depending on the fuel.
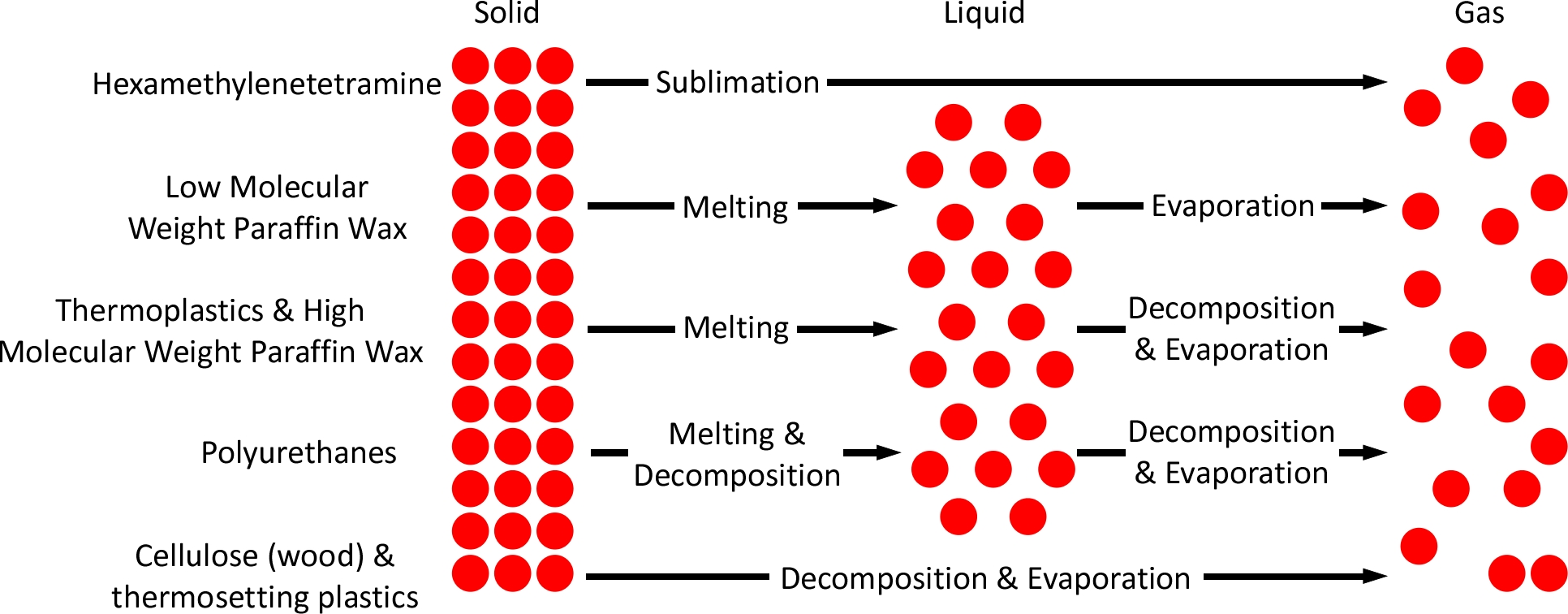
In addition to influencing the process of pyrolysis, resulting in a high mass fraction of flammable pyrolysis products, if temperature within the enclosure is above the autoignition temperature (AIT) of the mixture of air and fuel, it will ignite as soon as a flammable mixture is achieved in the boundary between the fuel and the gravity current (air) (Wu & Carvel, 2017)
If the temperature is below the AIT of the mixture of air and fuel, the increased oxygen concentration within the gravity current may increase the burning rate of smoldering fuel and potentially return that fuel to flaming combustion, providing a source of ignition that may ignite the fuel-air mixture when it reaches its flammable range (Zhao et al., 2021)
In their small scale experiments with polypropylene as a fuel source, Wu and Carvel (2017) identified that there was a minimum temperature (below the AIT) for the occurrence of a backdraft and that below this temperature backdrafts did not occur. When temperatures within the compartment were above the minimum temperature, but below the AIT, a higher fuel mass fraction was required. Wu and Carvel also speculated that the auto ignition temperature and critical temperature were likely to be highly fuel specific. Wu, Santamaria, and Carvel (2020) also identified the critical temperature for auto-ignition as a factor in occurrence of backdrafts.
Putting it all Together
The driving factors leading to a backdraft; mass fraction of fuel and oxygen, mixing at the interface between the gravity current and hot, buoyant, fuel rich smoke, temperature, and presence of ignition sources within the flammable zone of the fuel-air mixture must fall within a narrow range of conditions. Understanding these dynamics provides a basis for recognizing backdraft potential and understanding how the potential for a backdraft may be mitigated.
What’s Next
Next up will be a look at backdraft indicators, myth and reality along with alternative approaches to mitigate backdraft risk.
References
Bolliger, I. (1995). Full residential -scale backdraft. Retrieved May 28, 2025, from https://bit.ly/3FoDT48.
Carvel, R. & Wu, C. (2017). An experimental study on backdraught: the dependence on temperature, Fire Safety Journal, 91, pp. 320-326. doi: 10.1016/j.firesaf.2017.04.003.
Casey, J. (1968). The fire chief’s handbook (3rd ed). New York: The Ruben H. Donnelley Corporation.
Chen, A., Zhou, L., Liu, B., & Chen, W. (2011). Theoretical analysis and experimental study on critical conditions of backdraft, Journal of Loss Prevention in the Process Industries. 24, 632–637. doi:10.1016/j.jlp.2011.05.001.
Clark, W. (1974). Firefighting principles and practices. New York: Dunn Donnelly Publishing Corporation.
Fleischmann, C. (1994), Backdraft Phenomena. Final Report. Retrieved May 28, 2025, from https://bit.ly/4dG9T0s.
Gojkovic, D. (2000). Initial backdraft experiments. Retrieved May 26, 2025, from https://bit.ly/3Fyu1Vn.
Gottuk, D.; Williams, F.; & Farley, J. (1997). The development and mitigation of backdrafts: a full-scale experimental study. Fire Safety Science, 5, 935-936. doi: 10.3801/IAFSS.FSS.5-935
Steward, P. Dust and smoke explosions. National Fire Protection Association Quarterly, 7, 424-428.
Tsai, L. & Chiu, C. ( 2013) Full-scale experimental studies for backdraft using solid materials, Process Safety and Environmental Protection. 91, 202–212. doi:10.1016/j.psep.2012.05.007.
Weng, W. & Fan, W. (2003). Critical condition of backdraft in compartment fires: a reduced-scale experimental study, Journal of Loss Prevention in the Process Industries. 16, 19–26. doi:10.1016/S0950-4230(02)00088-8
Wooley, W. & Ames, S. (1975). The explosive risk of stored foamed rubber. Borehamwood, UK: Building Research Establishment.
Wu, C., Santamaria, S., & Carvel, R. (2019) Critical factors determining the onset of backdraft using solid fuels’ Fire Technology. doi: 10.1007/s10694-019-00914-9
Zabetakis, M. (1965). Flammability characteristics of combustible gases and vapors. Washington, DC: United States Department of the Interior, Bureau of Mines. Retrieved May 28, 2025, from https://bit.ly/4mSK01F.
Zhao, J., Li, Y., Li, J., Huang, Y., & Wu, J. (2021) Experimental study on the backdraft phenomenon of solid fuel. PLoS ONE, 16(8): doi: 10.1371/journal.pone.0255572.